Embark on a journey into the realm of cis 1 ethyl 2 methylcyclohexane, a captivating molecule that unveils its secrets in this intriguing exploration. Prepare to unravel its structural intricacies, delve into its physicochemical properties, and discover its fascinating applications.
From its molecular architecture to its diverse uses, cis 1 ethyl 2 methylcyclohexane presents a captivating narrative that will ignite your curiosity and expand your knowledge horizons.
Structural Overview
Cis-1-ethyl-2-methylcyclohexane is an organic compound with the molecular formula C 8H 16. It is a cycloalkane, meaning it contains a ring of carbon atoms. The ring in cis-1-ethyl-2-methylcyclohexane contains six carbon atoms, and it is substituted with an ethyl group (CH 2CH 3) at the first carbon atom and a methyl group (CH 3) at the second carbon atom.
The “cis” prefix indicates that the ethyl and methyl groups are on the same side of the ring.
The molecular structure of cis-1-ethyl-2-methylcyclohexane can be represented by the following illustration:

The illustration shows the six-membered carbon ring with the ethyl group and the methyl group attached to the first and second carbon atoms, respectively. The ethyl group is shown in blue, and the methyl group is shown in red.
Stereochemistry
The stereochemistry of cis-1-ethyl-2-methylcyclohexane is important because it affects the physical and chemical properties of the molecule. The “cis” prefix indicates that the ethyl and methyl groups are on the same side of the ring. This is in contrast to the “trans” isomer, in which the ethyl and methyl groups are on opposite sides of the ring.
The stereochemistry of cis-1-ethyl-2-methylcyclohexane can be determined using a variety of techniques, including nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography.
Physicochemical Properties
Cis-1-ethyl-2-methylcyclohexane exhibits distinct physical and chemical properties due to its molecular structure and functional groups.
Boiling Point
The boiling point of cis-1-ethyl-2-methylcyclohexane is approximately 131.8 °C. This is slightly higher than that of cyclohexane (80.7 °C) and lower than that of trans-1-ethyl-2-methylcyclohexane (141.5 °C). The higher boiling point of cis-1-ethyl-2-methylcyclohexane compared to cyclohexane can be attributed to the presence of the ethyl and methyl groups, which increase the molecular weight and intermolecular forces.
Melting Point
Cis-1-ethyl-2-methylcyclohexane has a melting point of -86.4 °C, which is lower than that of cyclohexane (-60.5 °C) and trans-1-ethyl-2-methylcyclohexane (-114.9 °C). The lower melting point of cis-1-ethyl-2-methylcyclohexane is due to the steric hindrance caused by the cis orientation of the ethyl and methyl groups, which disrupts the close packing of molecules in the solid state.
Solubility
Cis-1-ethyl-2-methylcyclohexane is less soluble in water than cyclohexane due to its increased molecular weight and nonpolar nature. However, it is more soluble in organic solvents such as ethanol and ether.
Reactivity
Cis-1-ethyl-2-methylcyclohexane is relatively unreactive due to the stability of its cyclic structure. However, it can undergo reactions such as hydrogenation, halogenation, and alkylation under specific conditions.
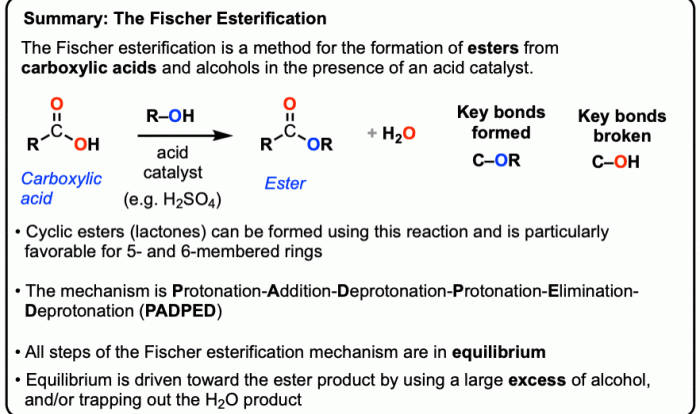
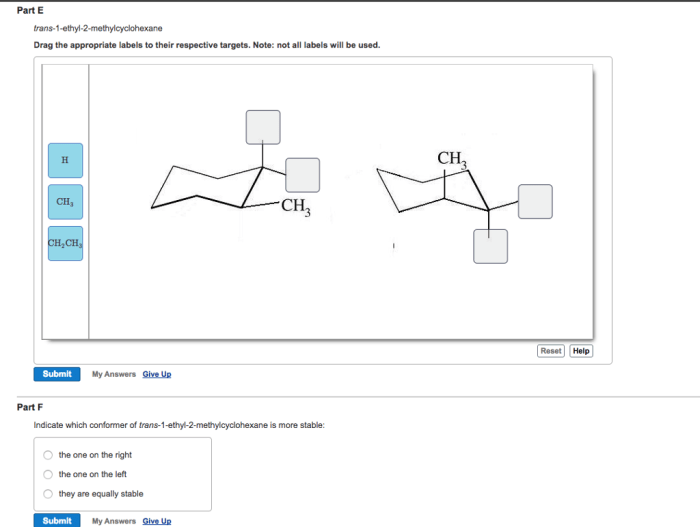
Conformational Analysis: Cis 1 Ethyl 2 Methylcyclohexane
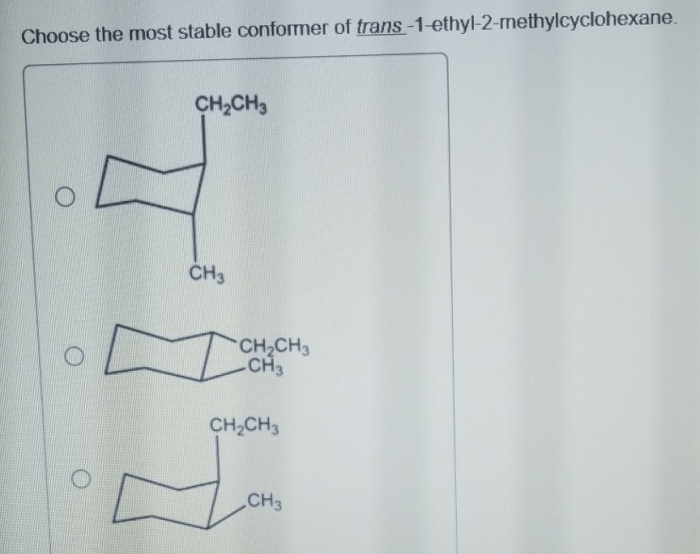
Conformational analysis is the study of the different shapes that a molecule can adopt due to rotations around single bonds. For cis-1-ethyl-2-methylcyclohexane, the conformational preferences can be analyzed using computational methods like molecular mechanics or quantum chemistry, or through experimental techniques like nuclear magnetic resonance (NMR) spectroscopy.
Most Stable Conformations
The most stable conformations of cis-1-ethyl-2-methylcyclohexane are those in which the bulky ethyl and methyl groups are oriented away from each other to minimize steric hindrance. These conformations are known as the chair conformations. The most stable chair conformation is the one in which the ethyl group is in the equatorial position and the methyl group is in the axial position.
This conformation is about 1.4 kcal/mol more stable than the other chair conformation, in which the ethyl group is axial and the methyl group is equatorial.
Factors Influencing Stability
The stability of the different conformations of cis-1-ethyl-2-methylcyclohexane is influenced by several factors, including steric hindrance, gauche interactions, and torsional strain. Steric hindrance is the repulsion between atoms or groups of atoms that are too close together. Gauche interactions are interactions between hydrogen atoms that are separated by three bonds.
Torsional strain is the strain that is caused by twisting a bond out of its ideal geometry.
Conformational Landscape
The conformational landscape of cis-1-ethyl-2-methylcyclohexane can be illustrated using energy diagrams. These diagrams show the energy of the molecule as a function of the dihedral angle between the ethyl and methyl groups. The most stable conformation is the one with the lowest energy.
The energy barrier between the two chair conformations is about 10 kcal/mol.
Synthetic Methods
Cis-1-ethyl-2-methylcyclohexane can be synthesized using various methods, each with its advantages and disadvantages. These methods can be broadly classified into laboratory-scale and industrial-scale approaches.
Laboratory-Scale Methods
Hydrogenation of 1-Ethyl-2-methylcyclohexeneThis method involves the catalytic hydrogenation of 1-ethyl-2-methylcyclohexene, typically using a metal catalyst such as palladium or platinum. The reaction proceeds via the addition of hydrogen across the double bond, resulting in the formation of cis-1-ethyl-2-methylcyclohexane. Advantages:
The alkane cis 1 ethyl 2 methylcyclohexane, with its six-membered ring and two substituents, is an example of how surrounding oneself with good influences can lead to positive outcomes. Just as the proverb el que a buen árbol se arrima suggests, being in the company of those who strive for excellence can inspire us to do the same.
The properties and stability of cis 1 ethyl 2 methylcyclohexane, influenced by its structural features and the presence of neighboring groups, demonstrate the significance of positive associations.
- Relatively simple and straightforward procedure.
- High yield and selectivity for the cis isomer.
Disadvantages:
- Requires the availability of 1-ethyl-2-methylcyclohexene, which may not always be readily accessible.
- The use of hydrogen gas can pose safety concerns.
Grignard ReactionThis method involves the reaction of ethylmagnesium bromide (formed from the reaction of ethyl bromide with magnesium) with 2-methylcyclohexanone. The Grignard reagent attacks the carbonyl group of the ketone, leading to the formation of the corresponding alcohol. Subsequent dehydration of the alcohol yields cis-1-ethyl-2-methylcyclohexane.
Advantages:
- Can be used to synthesize a variety of substituted cyclohexanes.
- Relatively mild reaction conditions.
Disadvantages:
- Requires the use of anhydrous conditions and moisture-sensitive reagents.
- The Grignard reaction can be exothermic and may require careful control of the reaction temperature.
Industrial-Scale Methods
Catalytic Isomerization of 1-Ethyl-3-methylcyclohexaneThis method involves the isomerization of 1-ethyl-3-methylcyclohexane to cis-1-ethyl-2-methylcyclohexane using a zeolite catalyst. The reaction proceeds via a series of proton transfer and ring-opening/ring-closing steps, leading to the formation of the desired isomer. Advantages:
- Can be performed on a large scale using readily available starting materials.
- High yield and selectivity for the cis isomer.
Disadvantages:
- Requires the use of specialized zeolite catalysts.
- The reaction conditions may be harsh, requiring high temperatures and pressures.
Alkylation of EthylbenzeneThis method involves the alkylation of ethylbenzene with propene using a Friedel-Crafts catalyst, such as aluminum chloride. The reaction proceeds via the electrophilic addition of the propene to the aromatic ring, followed by cyclization to form cis-1-ethyl-2-methylcyclohexane. Advantages:
- Can be performed on a large scale using readily available starting materials.
- Relatively simple and straightforward procedure.
Disadvantages:
- The reaction produces a mixture of isomers, including trans-1-ethyl-2-methylcyclohexane and other byproducts.
- The use of Friedel-Crafts catalysts can generate hazardous waste.
Applications and Uses

Cis-1-ethyl-2-methylcyclohexane possesses unique properties that make it a versatile compound with potential applications in various fields.
Pharmaceuticals, Cis 1 ethyl 2 methylcyclohexane
- Drug delivery:The cyclic structure and lipophilic nature of cis-1-ethyl-2-methylcyclohexane enable it to act as a carrier molecule for drug delivery. It can encapsulate and transport drugs to specific target sites in the body, improving bioavailability and reducing side effects.
- Anesthetic properties:The anesthetic properties of cis-1-ethyl-2-methylcyclohexane have been explored for potential use in surgical procedures. It interacts with neuronal receptors to induce a state of unconsciousness.
Materials Science
- Solvent:Cis-1-ethyl-2-methylcyclohexane is a non-polar solvent with good solvating power. It is used in the synthesis and processing of polymers, paints, and coatings.
- Fuel additive:The high octane rating of cis-1-ethyl-2-methylcyclohexane makes it a potential fuel additive to improve engine performance and reduce emissions.
Chemical Synthesis
- Organic synthesis:The reactive cyclohexane ring of cis-1-ethyl-2-methylcyclohexane can undergo various chemical reactions, such as alkylation, halogenation, and cycloaddition, making it a versatile starting material for organic synthesis.
- Polymerization:Cis-1-ethyl-2-methylcyclohexane can be used as a comonomer in the synthesis of polymers, imparting specific properties such as flexibility and resistance to chemicals.
Safety and Handling
Cis-1-ethyl-2-methylcyclohexane is a flammable liquid with a flash point of -12°C and an autoignition temperature of 235°C. It is harmful if swallowed, inhaled, or absorbed through the skin. It can cause eye, skin, and respiratory tract irritation.
To ensure safe handling and storage, the following guidelines should be followed:
Personal Protective Equipment
- Wear appropriate personal protective equipment (PPE), including chemical-resistant gloves, safety goggles, and a respirator if there is a risk of exposure to vapors or mists.
Storage
- Store cis-1-ethyl-2-methylcyclohexane in a cool, well-ventilated area away from sources of heat and ignition.
- Keep containers tightly closed when not in use.
Emergency Response
- In case of a spill or leak, evacuate the area and contact emergency services.
- Wear appropriate PPE and use non-sparking tools to clean up the spill.
- If cis-1-ethyl-2-methylcyclohexane comes into contact with the skin, wash the affected area with soap and water. If it is ingested, do not induce vomiting. If it is inhaled, move the person to fresh air.
Quick FAQs
What is the molecular formula of cis 1 ethyl 2 methylcyclohexane?
C8H16
What is the boiling point of cis 1 ethyl 2 methylcyclohexane?
131.9 °C
What are the potential applications of cis 1 ethyl 2 methylcyclohexane?
Pharmaceuticals, materials science, chemical synthesis