Unveiling the intricacies of acids, bases, and pH, this comprehensive worksheet provides a profound understanding of these fundamental chemical concepts. With acids bases and ph worksheet answers at the forefront, this guide embarks on an enlightening journey, unraveling the properties, types, and applications of these substances.
Delving into the realm of acids, we explore their defining characteristics, diverse classifications, and practical uses. Bases, their counterparts, reveal their unique properties, variations, and applications. pH, a crucial measure of acidity or alkalinity, takes center stage, shedding light on its significance, scale, and implications.
Introduction
Acids, bases, and pH are fundamental concepts in chemistry that play a crucial role in understanding various chemical reactions and processes. They are essential in many scientific disciplines, including biology, environmental science, and medicine.
An acid is a substance that donates protons (H+ ions), while a base is a substance that accepts protons. The pH of a solution measures its acidity or basicity on a scale from 0 to 14, with 0 being the most acidic and 14 being the most basic.
Understanding these concepts is vital for comprehending the behavior of chemical systems and their applications in various fields.
Properties of Acids, Acids bases and ph worksheet answers
- Sour taste
- React with metals to produce hydrogen gas
- Turn blue litmus paper red
- Conduct electricity in aqueous solutions
- Examples: Hydrochloric acid (HCl), sulfuric acid (H2SO4), acetic acid (CH3COOH)
Properties of Bases
- Bitter taste
- Slippery feel
- Turn red litmus paper blue
- Conduct electricity in aqueous solutions
- Examples: Sodium hydroxide (NaOH), potassium hydroxide (KOH), ammonia (NH3)
pH Scale
The pH scale measures the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while those with a pH above 7 are basic.
- Acidic solutions have a high concentration of H+ ions and a low concentration of OH- ions.
- Basic solutions have a low concentration of H+ ions and a high concentration of OH- ions.
Acids
Acids are chemical compounds that, when dissolved in water, release hydrogen ions (H+). They are characterized by their sour taste, corrosive nature, and ability to react with bases to form salts.
Acids can be classified into two main types: inorganic acids and organic acids.
Inorganic Acids
Inorganic acids are acids that do not contain carbon atoms. Some common examples of inorganic acids include:
- Hydrochloric acid (HCl): A strong acid used in metalworking, food processing, and laboratory applications.
- Sulfuric acid (H2SO4): A strong acid used in the production of fertilizers, batteries, and chemicals.
- Nitric acid (HNO3): A strong acid used in the production of explosives, fertilizers, and dyes.
- Phosphoric acid (H3PO4): A weak acid used in the production of fertilizers, detergents, and food additives.
Organic Acids
Organic acids are acids that contain carbon atoms. Some common examples of organic acids include:
- Acetic acid (CH3COOH): A weak acid found in vinegar and used as a preservative and flavoring agent.
- Citric acid (C6H8O7): A weak acid found in citrus fruits and used as a flavoring agent and preservative.
- Lactic acid (C3H6O3): A weak acid produced by bacteria during fermentation and used in the production of yogurt, cheese, and other fermented foods.
- Oxalic acid (C2H2O4): A weak acid found in rhubarb and sorrel and used as a bleaching agent and cleaning agent.
Bases
Bases are substances that release hydroxide ions (OH-) when dissolved in water. They are typically characterized by a bitter taste, a slippery feel, and the ability to turn red litmus paper blue. Bases can be classified into two main types: strong bases and weak bases.
Strong bases are those that completely dissociate in water, releasing all of their hydroxide ions. Examples of strong bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2). Strong bases are highly corrosive and can cause severe burns.
Weak bases are those that only partially dissociate in water, releasing only a small fraction of their hydroxide ions. Examples of weak bases include ammonia (NH3), sodium bicarbonate (NaHCO3), and calcium carbonate (CaCO3). Weak bases are less corrosive than strong bases, but they can still cause skin irritation.
Bases are used in a wide variety of applications, including:
- Cleaning products: Bases are used in many cleaning products, such as soaps, detergents, and oven cleaners. They help to remove dirt and grime by breaking down organic matter.
- Food preparation: Bases are used in the preparation of many foods, such as baking soda (sodium bicarbonate), which is used to make baked goods rise. Bases are also used to make certain types of cheese, such as mozzarella and cheddar.
- Medicine: Bases are used in a variety of medical applications, such as antacids, which are used to neutralize stomach acid, and eye drops, which are used to treat eye infections.
- Industry: Bases are used in a variety of industrial applications, such as the production of paper, textiles, and fertilizers.
pH
pH is a measure of the acidity or basicity of an aqueous solution. It is defined as the negative logarithm of the molar concentration of hydrogen ions in the solution.
The pH scale ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while solutions with a pH above 7 are basic. The pH of a solution can be measured using a pH meter or with pH paper.
pH Scale and Applications
The pH scale is used in a variety of applications, including:
- Chemistry: to determine the acidity or basicity of solutions and to study chemical reactions
- Biology: to study the pH of biological fluids, such as blood and urine
- Environmental science: to study the pH of water and soil
- Medicine: to diagnose and treat medical conditions, such as acid reflux and kidney stones
Examples of pH Values
The pH values of some common substances are:
| Substance | pH |
|---|---|
| Hydrochloric acid | 1 |
| Lemon juice | 2 |
| Orange juice | 3 |
| Coffee | 5 |
| Pure water | 7 |
| Baking soda solution | 9 |
| Ammonia | 11 |
| Bleach | 13 |
Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of protons (H+) between reactants. These reactions play a crucial role in various chemical and biological processes.
There are three main types of acid-base reactions:
Neutralization Reactions
Neutralization reactions occur between an acid and a base, resulting in the formation of a salt and water. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H2O):
“`HCl + NaOH → NaCl + H2O“`
Proton Transfer Reactions
Proton transfer reactions involve the transfer of a proton from an acid to a base. For example, the reaction between acetic acid (CH3COOH) and water produces hydronium ions (H3O+) and acetate ions (CH3COO-):
“`CH3COOH + H2O → H3O+ + CH3COO-“`
Hydrolysis Reactions
Hydrolysis reactions involve the reaction of a salt with water to produce an acid and a base. For example, the hydrolysis of sodium acetate (CH3COONa) produces acetic acid (CH3COOH) and sodium hydroxide (NaOH):
“`CH3COONa + H2O → CH3COOH + NaOH“`
Applications of Acids, Bases, and pH: Acids Bases And Ph Worksheet Answers
Acids, bases, and pH play crucial roles in various aspects of everyday life. They find applications in industries, medicine, research, and many other fields.
Understanding the properties and behavior of acids and bases is essential for optimizing processes, ensuring safety, and achieving desired outcomes in numerous applications.
In Industries
- Food and Beverage Production:Acids and bases are used to adjust pH levels, enhance flavors, and preserve food products.
- Textile Manufacturing:Acids are employed in dyeing processes to fix colors and improve fabric quality.
- Metalworking:Acids are used for metal cleaning, etching, and electroplating.
- Chemical Production:Acids and bases are essential reagents in the synthesis of various chemicals, including pharmaceuticals and fertilizers.
In Medicine
- Drug Development:Acids and bases are used to modify the solubility, absorption, and efficacy of drugs.
- Medical Diagnosis:pH measurements are used in blood tests, urine analysis, and other diagnostic procedures.
- Treatment of Diseases:Acids and bases are used as therapeutic agents to neutralize stomach acid, treat skin conditions, and dissolve kidney stones.
In Research
- Biochemistry:Acids and bases are used to study enzyme reactions, protein structures, and other biochemical processes.
- Environmental Science:pH measurements are used to assess water quality, soil acidity, and the impact of pollutants on ecosystems.
- Materials Science:Acids and bases are used to etch surfaces, modify materials, and create new compounds.
Importance of pH Control
pH control is crucial in many applications to ensure optimal conditions, prevent damage, and maintain stability.
- Industrial Processes:pH control is essential for corrosion prevention, chemical reactions, and product quality.
- Biological Systems:pH levels are critical for enzyme activity, cell viability, and overall health of living organisms.
- Environmental Protection:pH control is necessary to maintain water quality, prevent acid rain, and mitigate the impact of industrial pollution.
Worksheet Answers
The following table provides answers to the questions presented in the acids, bases, and pH worksheet. Each answer is accompanied by a detailed explanation to enhance understanding.
Question 1: Define an acid.
An acid is a substance that donates hydrogen ions (H+) when dissolved in water. Acids typically have a sour taste and can react with bases to form salts and water.
Question 2: What is the pH of a neutral solution?
The pH of a neutral solution is 7. A neutral solution has an equal concentration of hydrogen ions (H+) and hydroxide ions (OH-).
Question 3: What is the relationship between pH and acidity?
pH and acidity are inversely related. A lower pH indicates a higher acidity, while a higher pH indicates a lower acidity. This is because pH measures the concentration of hydrogen ions (H+) in a solution, and higher concentrations of H+ ions correspond to greater acidity.
Question 4: Give an example of a strong acid.
Hydrochloric acid (HCl) is an example of a strong acid. Strong acids completely dissociate in water, releasing all their hydrogen ions.
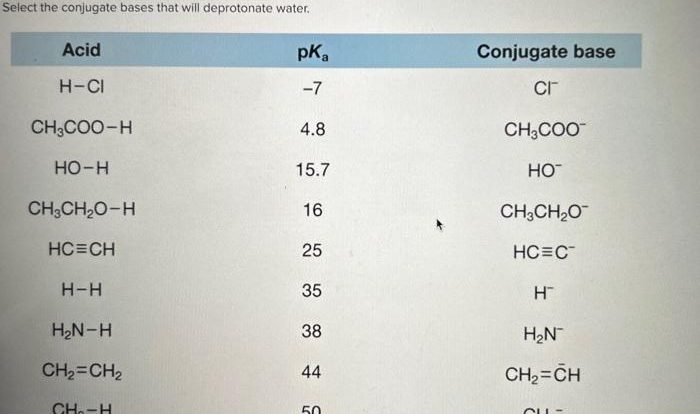
Question 5: What is the conjugate base of a weak acid?
The conjugate base of a weak acid is the species that results from the dissociation of the acid. For example, the conjugate base of acetic acid (CH3COOH) is acetate ion (CH3COO-).
Question 6: What is the difference between a buffer solution and a normal solution?
A buffer solution is a solution that resists changes in pH when small amounts of acid or base are added. A normal solution is a solution with a specific concentration of solute, expressed in equivalents per liter.
Question 7: What are some applications of acids, bases, and pH?
Acids, bases, and pH have numerous applications in various fields, including:
- Industry: Acids and bases are used in the production of fertilizers, plastics, and pharmaceuticals.
- Medicine: Acids and bases are used to regulate pH levels in the body and to treat various diseases.
- Environmental science: Acids and bases are used to monitor and control pollution.
FAQ
What is the difference between an acid and a base?
Acids release hydrogen ions (H+) in water, while bases release hydroxide ions (OH-) in water.
What is the pH scale?
The pH scale measures the acidity or alkalinity of a substance, ranging from 0 (highly acidic) to 14 (highly alkaline), with 7 being neutral.
What are some common applications of acids and bases?
Acids are used in batteries, fertilizers, and food preservation, while bases are used in soaps, detergents, and antacids.