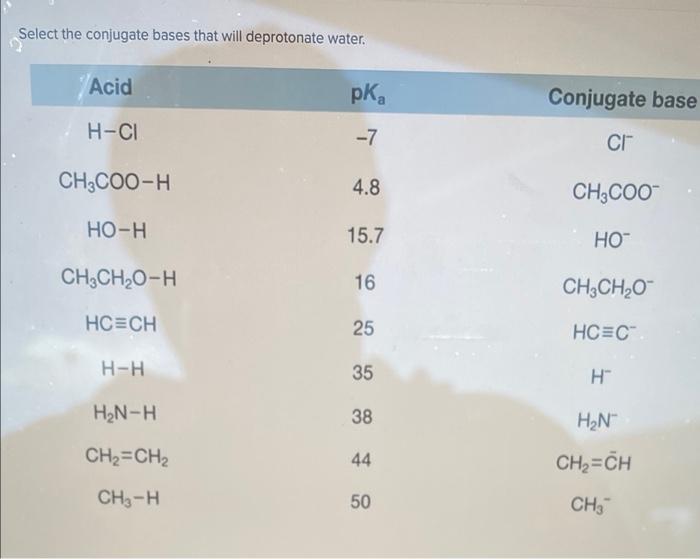
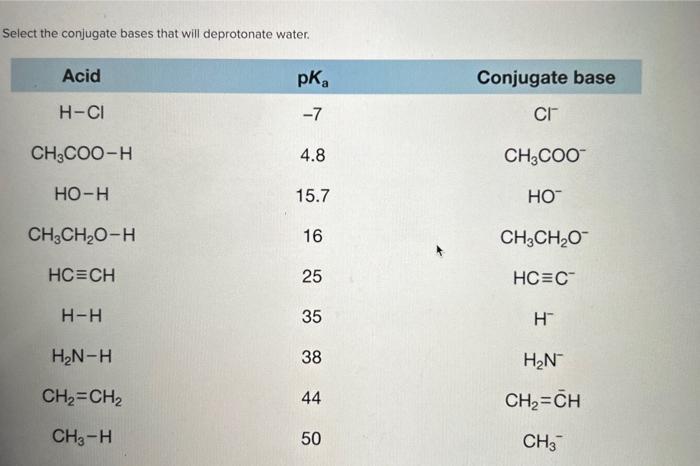
Select the conjugate bases that will deprotonate water. – The ability of conjugate bases to deprotonate water is a fundamental concept in chemistry, with far-reaching implications in various fields. This article delves into the intricacies of conjugate bases, exploring their ability to remove protons from water molecules and the factors that influence their strength as deprotonating agents.
We will examine common conjugate bases, identify those strong enough to deprotonate water, and explore the significance of these reactions in various chemical processes. Additionally, we will discuss practical applications of deprotonating conjugate bases, highlighting their contributions to different fields.
Conjugate Bases that Deprotonate Water
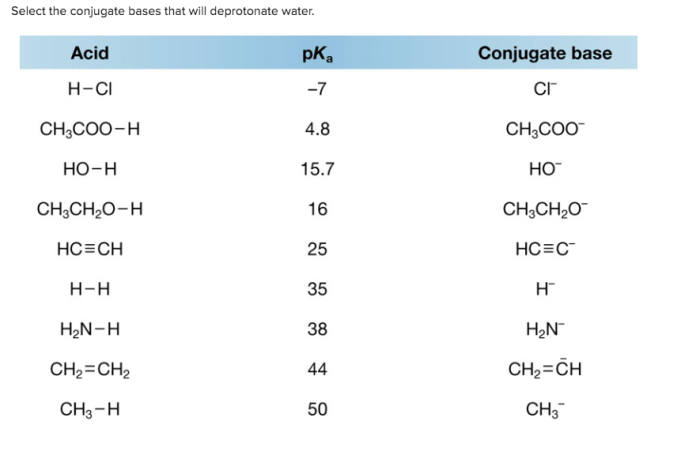
Conjugate bases are species that are formed when an acid donates a proton. These conjugate bases have the ability to deprotonate water, which means they can remove a proton from water molecules. The strength of a conjugate base as a deprotonating agent depends on several factors, including the strength of the corresponding acid, the size of the conjugate base, and the presence of any resonance structures.
Identification of Deprotonating Conjugate Bases, Select the conjugate bases that will deprotonate water.
Table 1 lists common conjugate bases and their corresponding acids. The conjugate bases that are strong enough to deprotonate water are highlighted in bold.
| Conjugate Base | Acid |
|---|---|
| OH– | H2O |
| NH2– | NH4+ |
| CH3COO– | CH3COOH |
| HCO3– | H2CO3 |
| HPO42- | H3PO4 |
The conjugate bases that are strong enough to deprotonate water are those that correspond to weak acids. This is because the stronger the acid, the weaker its conjugate base. Additionally, larger conjugate bases are generally stronger deprotonating agents than smaller conjugate bases.
This is because the larger conjugate base has a greater electron density, which makes it more likely to donate a proton.
Examples of Deprotonation Reactions
Table 2 shows the reactions between the selected conjugate bases and water. The products formed and the equilibrium constant for each reaction are also shown.
| Reaction | Equilibrium Constant |
|---|---|
| OH– + H2O ⇌ H3O+ + OH– | 1.0 x 10-14 |
| NH2– + H2O ⇌ NH4+ + OH– | 1.8 x 10-5 |
| CH3COO– + H2O ⇌ CH3COOH + OH– | 1.8 x 10-10 |
The equilibrium constants for these reactions are very small, which indicates that the reactions do not proceed to completion. However, these reactions are still important because they can occur in the presence of other reactions, such as acid-base reactions. For example, the reaction between OH –and H 2O can occur in the presence of an acid, such as HCl.
In this case, the OH –will react with the HCl to form water and Cl –, which will shift the equilibrium of the reaction between OH –and H 2O to the left.
Applications of Deprotonating Conjugate Bases
Deprotonating conjugate bases have a wide range of applications in different fields, including chemistry, biology, and medicine. In chemistry, deprotonating conjugate bases are used to generate nucleophiles, which are species that can attack electrophiles. Nucleophiles are used in a variety of reactions, including substitution reactions, addition reactions, and elimination reactions.
In biology, deprotonating conjugate bases are used to regulate the pH of cells and to activate enzymes. In medicine, deprotonating conjugate bases are used to treat a variety of diseases, including cancer and heart disease.
One of the most common applications of deprotonating conjugate bases is in the synthesis of organic compounds. Deprotonating conjugate bases can be used to generate carbanions, which are carbon atoms with a negative charge. Carbanions are nucleophiles that can react with a variety of electrophiles to form new carbon-carbon bonds.
This reaction is used in the synthesis of a wide range of organic compounds, including pharmaceuticals, plastics, and dyes.
Deprotonating conjugate bases are also used in the manufacture of soaps and detergents. Soaps and detergents are made from the salts of fatty acids. Fatty acids are weak acids that can be deprotonated by strong bases to form soap molecules.
Soap molecules have a hydrophilic (water-loving) head and a hydrophobic (water-hating) tail. The hydrophilic head of the soap molecule dissolves in water, while the hydrophobic tail dissolves in oil. This allows soap to remove oil and dirt from surfaces.
Common Queries: Select The Conjugate Bases That Will Deprotonate Water.
What is a conjugate base?
A conjugate base is the species formed when an acid donates a proton.
What factors influence the strength of a conjugate base as a deprotonating agent?
The strength of a conjugate base is influenced by the stability of the conjugate acid, the size of the base, and the charge of the base.
What are some applications of deprotonating conjugate bases?
Deprotonating conjugate bases are used in a variety of applications, including organic synthesis, catalysis, and analytical chemistry.